Certain metals like silver, copper, Aluminum lose their electrons in the outermost shell very easily, and such electrons are called free electrons. Due to the loss of negatively charged electrons the neutral atom is left with an excessive positive charge and is called as positive ion represented by circle with a + sign in it. The process by which an atom loses an electron is called as ionization.
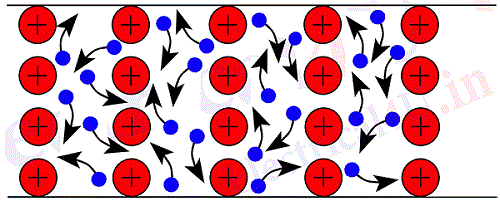
These free electrons of a metal move about haphazardly in all directions from atom to atom. At any instant there will be a number of free electrons and positively charged ions. There is always a tendency for the positive ion to attract the negatively charged electron to form a neutral atom again, and so the free electrons move away to be attached to other ions. This process of attaching and detaching of an electron goes on at a very large scale and the average velocity of movement of electrons in any direction is zero. Fig.1 shows a random movement of electrons in a conductor. This random movement of electrons can be compared with the random movement of gas particles in closed room.
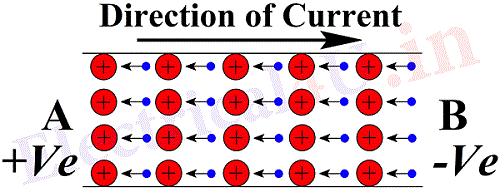
If now a door and an opposite window of the room are opened, and if there is a difference of pressure between the room and the atmosphere, the gas particles will be seen to have a definite direction of motion (which is always from higher pressure to a lower pressure). This movement of the gas particles in a definite direction is called an air current. Similarly when a certain electrical pressure or potential is applied to such metal at the two ends, the movement of electrons drift only in one direction. The drift of electrons in a conductor in one direction is called as the electric current. Fig.2 shows the movement of electrons when a potential is applied to the conductor. The end A is at a higher potential or the positive end while the end B is negative. The direction of movement of electrons is from B to A due to the reason that the electrons have a negative charge and are attracted by the positive end. But, conventionally, the direction of current is said to be in the opposite direction to that of the direction of the drift of the electrons, or the direction of current is from higher potential to the lower potential.
Ampere – It has been said that the electric current is a drift of electrons. The strength of the electric current is measured in amperes and one ampere consist of movement of 6.26 x 1018 electrons at any section of the conductor. Thus an ampere is the rate of movement of electrons.
International Ampere – For the international definition of ampere the Standards Committee utilized the chemical effect of current flowing under definite conditions and defined it as that steady current which when flowing through a solution of Copper Sulphate Deposit 0.0003293 gram of copper on the cathode in one second.
Or, the international ampere may be defined as that steady current which when flowing under definite conditions in a solution of silver nitrate, deposits silver at the rate of 0.001118 gram per second.
Quantities of Current – Before explaining the quantity of current it is good to consider first a similar analogy of water flowing in a pipe. The rate of flow of water in the pipe can well be compared with the rate of electrons passing through any section of the conductor. The quantity of water flowing at any section of the pipe in a given time is equal to the multiplication of the rate of water flowing there and time. So, also, the quantity of current or electricity passing through any section of the conductor is the product of rate of movement of electrons at any point in the conductor and time and its unit is called Coulomb. If I is the current strength, then quantity of electricity Q in t seconds is given as Q = It
International Coulomb – It is that quantity of electricity which liberates 0.0003293 gram of copper from a solution of silver nitrate in one second.
Ampere-hour – It is that quantity of electricity which is conveyed by one ampere in one hour.
So 1 ampere-hour = 3600 coulombs.
