It has been observed that when a copper rod is rubbed with silk is not electrified and the rod is electrified only when it is held in an ebonite handle.
Also the glass rod with Silk is electrified only in the portion which is rubbed in the very beginning. As the copper rod allows electricity to flow through it and due to this reason it is called a conductor. But the glass and ebonite rod do not allow the electricity to flow through them and are called as insulators. Thus conductors and insulators may be defined as under:
Those substances, which offer least resistance to flow of electrons or electricity are called conductors and the substances offering high resistance to the movement of electrons are called insulator.
The following table gives the properties of the different insulating materials used:-
| Material | Dielectric strength | Rupturing strength kV per cm. | Permissible operating temperature 0C | Thermal conductivity Watt per cm. Per 10 |
| Air | 1.0 | 30 | – | 0.00025 |
| Asbestos | – | 20 | 450 | 0.0018 |
| Electrical cardboard | 3.0 | 90 to 130 | 90 | 0.0014 to 0.0025 |
| Mica | 6.7 | 1000 | 500 | 0.0036 |
| Paper | 2.5 to 3 | 80 to 90 | 90 | 0.0013 |
| Porcelain | 5.5 to 6.6 | 100 to 200 | – | 0.01 |
| Press palm | – | 13.5 | 90 | 0.0017 |
| Transformer oil | 2.2 | 70 to 120 | 95 | 0.0012 to 0.0017 |
| Varnished cloth | 3.0 to 4.0 | 240 to 650 | 150 | 0.0025 |
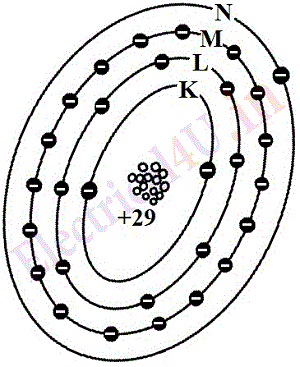
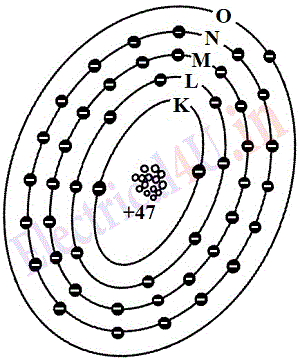
According to the electron theory, the atomic structure of conductors is such that one or more electrons in the outermost shells of these atoms are very weakly bound to the atom. As an example figure 1.4 represent the atomic structures of copper(Cu) and silver(Ag). The copper atom has 29 electrons, its M shell is complete and N shell has 1 electron, while silver atom has 47 electrons, it’s N shell is not complete but has 18 electrons, and also there is 1 electron in O shell. Is electron in the outermost shell is loosely bound which makes both copper and silver as conductors.
There are four conductors which can be used for conducting electrical energy. They are:-
- Silver
- Copper
- Aluminium
- Iron
Conductors are used for power transmission and interior wiring purpose because of their low resistance. In addition to these, German Silver, lead and various alloys such as maganin, constantan, nichrome etc., are used for electrical appliances such as fuses, instrument coils, heaters etc. but these are never used for transmission of electrical energy or fir power purposes because of their high resistance.
Silver : Made out very good conductor, but it is a precious metal so its industrial use in much Limited.
Copper : it is the next best conducted which is exclusively used electrical industry as overhead lines, cables, busbar Electromagnet, motor winding etc. It has the great advantage that it is reasonably cheap and is quite ductile. Moreover its specific resistance is quite low; but it much depends upon the purity and the physical treatment it has undergone e.g., the conductivity of the ordinary copper obtained by smelting process is only 50% that of electrolytically refined copper.
The copper conductor mostly used is of two types:-
a) hard drawn & b) Annealed.
The hard drawn copper conductor is made by bye-bye drawing eat gold through dies which make it much stronger in tensile strength but ductility is reduced full stop so the hard drawn copper conductor is used in overhead lines and trolley wires full stop the hard drawn wires can be made soft and ductile by heating it to a high temperature and allowing it to cool slowly. The process is called annealing and the conductors so produced are called as annealed. The annealed copper conductors are used in manufacture of winding wires, strips etc. The resistivity of hard and copper is 1.7 μ Ω per centimetre cube while that of annealed copper is 1.72 μ Ω per centimetre cube.
Aluminium : The electrical conductivity of it is approximately 60% that of copper. Because of its light weight it is mostly used in overhead transmission line. Its use in the underground cable is restricted has the jointing of aluminium cable is quite different if not impossible.
Iron : The use of it is restricted only to transmission lines of very long span where copper conductor is not mechanically very strong. The Steel wires have resistance of six to eight times that of annealed copper. In electric traction Steel rails are used as a written conductor. Ordinary Steel rails have a resistance of 11 to 13 times that of copper. Steel wires may be used with great advantage in Telegraph and telephone circuits change hai the distance is not a serious disadvantage.
The conductors can be divided the following three types:-
(1) metallic conductors: As explain above, the current in metals is conducted by the movement of electrons and there is no movement of the material itself.
(2) Electrolytic conductors: These are solutions of acids or alkalis in water full stop in this conductors, there is a movement of material with the movement of electrons.
(3) Gaseous conductors: The gas is decomposed into negative ions and positive ions and with the conduction of electricity these ions move.
The conductor and insulator are mostly affected by temperature. The resistances of most of the conductors increase (except carbon) with increase of temperature thus making good conductors as worse conductors. The insulating power of insulators also decrease with increase of temperature.
