There are several which have been advanced to prove the phenomena of electrification.
(1) Benjamin Franklin’s One-Fluid Theory. In 1749 Bar Franklin suggested that the electrification of bodies is due invisible fluid beyond human concepts which combines with them in different degrees. When the body is neutral it has a certain quantity of fluid. The body becomes positively charged if there is an excess of this fluid and becomes negatively charged if the quantity electric fluid decreases below its normal quantity (which makes it neutral). Thus before rubbing, the ebonite rod is neutral and it is rubbed with fur a certain amount of fluid passes from the rod to the fur so that the rod becomes negatively charged and the fur becomes positively charged but the total quantity of fluid remains the same.
This theory emphasizes that there are only two states of electricity and not two kinds of electricity. This theory did not survive long since it could not explain electrical induction and many other electrical phenomena.
(2) Symmer’s Two-Fluid Theory. According to Symmer, the electrification is due to two electric fluids, positive and negative. A neutral body possesses these fluids in equal quantity. When two bodies (ebonite and flannel) are rubbed the negative fluid goes to one (to ebonite) and the positive fluid goes to the other (to flannel).
This theory undoubtedly proves that two similar fluids repel each other and two unlike fluids attract each other and it also proves that there is a simultaneous development of two kinds of electrification.
This theory could not hold ground for long since it also lacked explanations about many facts of electricity.
(3) Modern Theory. The modern theory is also known as electron theory. This theory not only explains about attraction, repulsion, and two kinds of electrification in electrostatics, but also gives clear conception of voltage resistance, insulation, induced voltages and magnetism.
To follow this theory, consider that the whole of the universe is composed of two things; one is matter and the other is energy.
Matter may be defined as anything which possesses weight and occupies space and can be in any of the three forms: solid, liquid or gaseous. Matter consists of three ingredients:
(i) Positrons. These are particles having positive charge and are very light in weight.
(ii) Neutrons. These are particles having no charge, are neutral and they practically contribute towards whole of the weight of the matter.
(iii) Electrons. These have opposite charge to that of positrons or these possess negative charge and are also very light in weight.
All matter, according to the electron theory, is fundamentally the same; thus copper, iron, aluminum, etc., all have the same basic ingredients (positrons, neutrons and electrons), but arranged in a different manner. In other words, if the structure of aluminum is examined it will be found to have a certain specified number of positrons, neutrons and electrons arranged in a definite manner, and if the structure of iron is examined it will also have the positrons, neutrons and electrons but their number will be different, arranged in a different manner.
A matter can said to be composed of small particles called ‘atoms’; and the atom itself is composed of positrons, neutrons and electrons arranged in a particular fashion known as the structure of an atom.
The atom has a nucleus at the center, which consists of positrons and neutrons. As one positron and one neutron get together it is called one proton. As all elements have more neutrons than positrons, so the positrons do not exist themselves, but form protons and hence the nucleus has protons and excess of neutrons. Since the positron having a positive charge combines with a neutron having no charge, so the charge of proton is positive.
The protons and neutrons are closely packed together and form one group called the nucleus. The diameter of this closely packed nucleus is of the order of 10-12 cm.
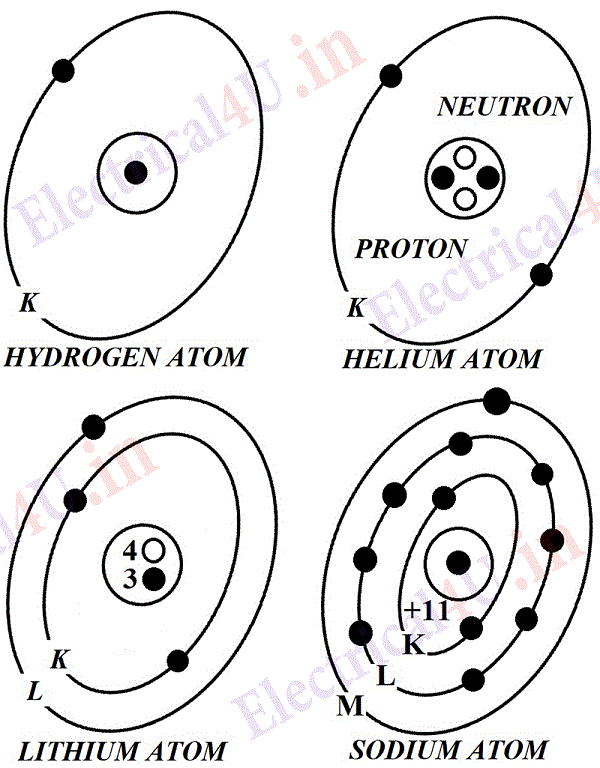
Around this nucleus are arranged orbits of electrons, in an elliptical fashion. The approximate diameter of this elliptical is of the order of 10-8 cm. Hence, the diameter of the electron orbit is about ten thousand times as great as that of the nucleus and the space in between them is empty. The first orbit or K shell around the nucleus may have one or two electrons but not more than 2; the second shell may have from one to eight, the third orbit or M shell may have 18 electrons and the N shell has maximum number of 32 electrons.
The simplest atom is that of hydrogen which has got 1 electron and 1 proton, and the atom of helium has 2 electrons in the K shell while the nucleus has 2 neutrons and 2 protons as shown in Fig. 1. Lithium has three electrons arranged as 2 in K shell and 1 in L shell and 3 protons and 4 neutrons, while sodium has 11 electron protons. Similarly the atomic structures of other substances can be represented.
The important properties of the atomic structure are:
Numerical:- The mass of proton and neutron is almost same while the mass of each electron is 1/1840 times that of a proton or neutron which shows that the mass of an atom is only due to the nucleus, From Avogadro’s hypothesis, the number of atoms in a gram atom is 6.03 x 1023 (actually its mass is 1.008 gm.).
So mass of 1 atom of hydrogen
= 1.008/(6.03*1023)
= 1.67*10-24 gm.
= 1.67*10-27 kg.
The mass of 1 electron
= (1.67*10-27)/1840
= 9.11*10-31 kg.
The charge attained by the electron is the basic practical unit; but since it is too small unit, a bigger practical unit called coulomb is used.
Also it has been practically observed that, to deposit 1 molecule gram of hydrogen one requires 96493.7 coulombs.
So, charge on each electron
= 96493.7/(6.03*1023)
= 1.602*10-19 coulombs.
The charge of proton is the same as that of electron
= 1.602*10-19 coulombs.
The charge and mass of an electron, proton and neutron can be summarized as:
| Name of atomic ingredient | Electric charge in coulomb | Mass in Kilogram |
| Electron | – 1.602 × 10-19 | 9.11 × 10-31 |
| Proton | + 1.602 × 10-19 | 1.67 × 10-27 |
| Neutron | 0 | 1.67 × 10-27 |
(ii) It has been established, that, the nucleus and the electrons form a miniature solar system. The nucleus spins on its own axis. The negatively charged ingredient of an atom, i.e. electrons, are arranged in shells. Each of the electron rotates around the nucleus in an elliptical ring; after one complete rotation the plane of the electron shift a few degrees so that now it will form another new ring (shown dotted); and after its completion another ring will be formed, then another and so on and it will trace a sphere.
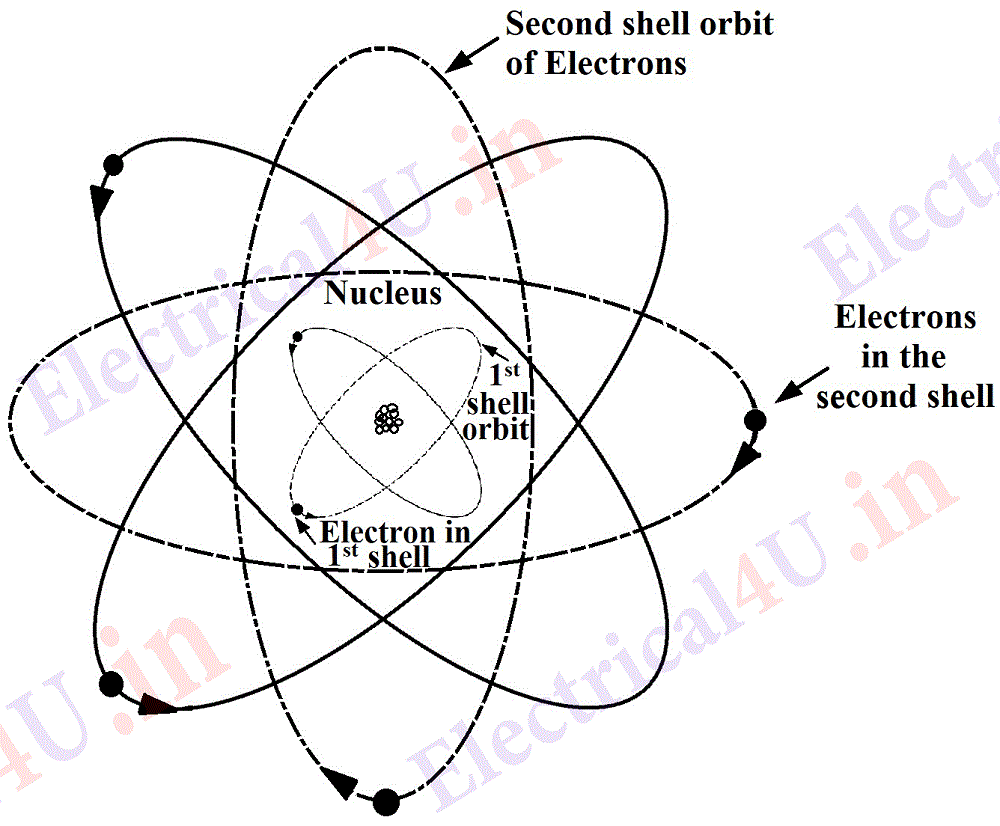
Moreover in a shell some of the electrons move in a clockwise direction and the others move in an anti-clockwise direction as shown in Fig.2. Thus the electrons move around the nucleus and rotate on their own axes also and it quite conforms to the solar system with the planets moving round the sun as the center and revolving on their own axes.
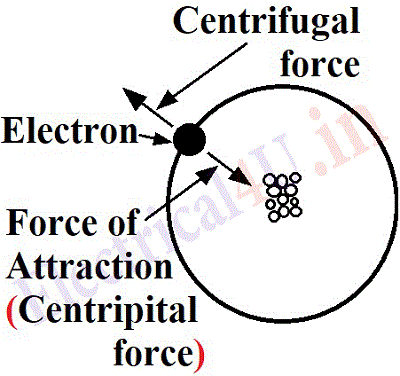
(iii) The electrons rotating in elliptical orbits around the nucleus experience a centrifugal force, also they experience a force of attraction (centripetal force) from the nucleus. Under the influence of these two forces, the electrons retain their circular path. The force of attraction is inversely proportional to the square of the distance between the electrons and the nucleus. The electrons in the first shell will experience a maximum force of attraction. The electrons in the second shell will be farther away from the nucleus, so attraction will be less ; further the electrons in the second shell move with lesser speed resulting in a lesser centrifugal force and thus there always remain a balance be on an electron.
As has been stated earlier, all substances are composed of matter and energy. Energy can be defined as the capacity to do work. There is a law of conservation of energy, according to which energy can neither be created nor it can be destroyed; but it can be converted from one form to another. For example, water from sea rises up in vapor form due to heat energy of the sun and forms clouds; the clouds due to their high position possess potential energy, and when the rain falls, this water forms streams, waterfalls and then rivers. Thus the potential energy is converted into kinetic energy. This kinetic energy may be used to turn the wheel of a turbine, thus changing kinetic energy to mechanical energy; and if an alternator is coupled to the turbine, the mechanical energy is converted into electrical energy which, in turn, can again be converted to heat energy.
The electrons in the structure of an atom do not always remain in the same atom, but can be detached from it when a certain energy is supplied to it. Thus when silk is rubbed on a glass rod, due to the friction (or heat energy), the electrons from the glass rod pass to the silk. This interchanging of electrons leaves the glass with excess of protons and positive charge of protons predominates. The glass becomes positively charged and the silk now possesses excess number of electrons than the normal, so it becomes negatively charged. The energy supplied to displace the electrons from the glass is converted in the form of field of force surrounding the glass rod.
