1. Bodies with like charge repel each other and bodies with unlike charge attract each other. i. e. If two positively charged bodies are placed near each other, they repel each other while if one is positively charged and other is negatively charged, they attract each other. The force of attraction or repulsion between these bodies will depend on the amount of charge and distance between the bodies.
2. Coulomb’s law states that, the force between two charged bodies i. e. attraction or repulsion between the two electrically charged bodies is directly proportional to the product of magnitudes of two charges and inversely proportional to the square of the distance between two charged bodies.
The relation is given by;
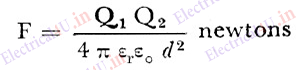
Where Q1 and Q2 are charges on the body 1 and body 2 in coulombs.
ε0 is the permittivity of free space or vacuum (approx. in air) =8.854187817…×10−12
εr is relative permittivity of the medium if the charges are in certain medium other than air.
d is the distance between two charged bodies in metre.
