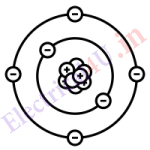
Atom is the smallest particle of matter and it it consist of three main part. Protons, neutrons and electrons. The protons and neutrons are normally intimate with each other and form the nucleus of the atom. The electrons are particles rotating in the orbit around the nucleus like planets around the sun. The number of protons, neutrons and electrons in the various elements are different. Protons are positively charged particles, electrons are negatively charged while neutrons have no electric charge. Following are the value their mass and charge.
| Mass | Charge | |
| Electron | 9.109 * 10-31 | -1.602 * 10-19 |
| Proton | 1.673 * 10-27 kg | 1.602 * 10-19 |
| Neutron | 1.673 * 10-27 kg | No charge |
Normally atoms are not electrified(neutral) since positively charged protons of any atom is exactly equal to the negatively charged electrons. If the electron from the outermost orbit is removed then body will be positively charged, if the electron is added to the atom body we will be negatively charged.
The process of removing and adding the electrons to the neutral atom is called ionization and the atom is called an ion. When the glass and Silk surface are in intimate contact, electrons of the surface of the glass are captured by the surface of the silk. In this process, glass becomes positively charged and silk becomes negatively charged.
